| Sign In | Join Free | My frbiz.com |
|
- Home
- Products
- About Us
- Quality Control
- Contact Us
- Get Quotations
| Sign In | Join Free | My frbiz.com |
|
Brand Name : Biovantion
Model Number : BVTE0152
Certification : ISO13485
Place of Origin : China
MOQ : 10
Price : Negotiable
Payment Terms : TT 100% PAYMENT
Supply Ability : 100000
Delivery Time : 7-15 days
Packaging Details : CARTON
Sample Type : Serum, Plasma,
Sensitivity : High
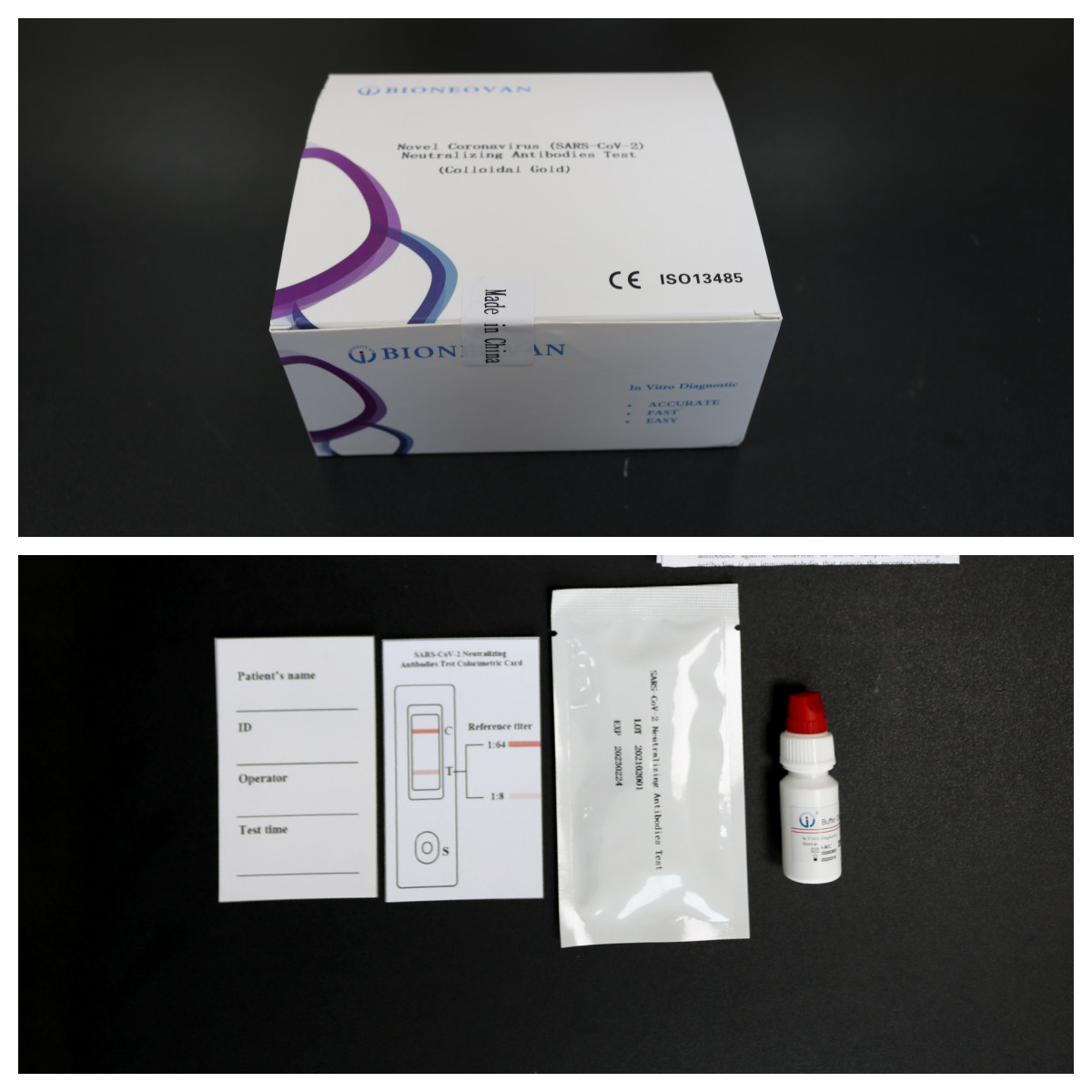
Product Name : SARS-CoV-2 Elisa Test
Applications : IVD
Country of Origin : China
Kit Size : 96 Tests
Shelf Life : 18 Months
Manufacturer : Biovantion
Method : ELISA Method
The SARS-CoV-2 Diagnostic Test Kit is designed for the rapid and accurate detection of the SARS-CoV-2 virus, which causes COVID-19. This test kit is intended for use in detecting the presence of the virus in clinical specimens, aiding in the diagnosis of COVID-19 and supporting public health efforts to manage and control the spread of the disease.
Storage and Stability:
Safety Information:
Ordering Information:
For more information or to place an order, please contact [Company Name] at [Company Contact Information] or visit our website at [Company Website].
Regulatory Status:
Performance Characteristics:
| Kit Size | 96 Tests |
| Test Type | ELISA |
| Country of Origin | China |
| Specificity | High |
| Format | Kit |
| Shelf Life | 18 Months |
| Specimen | Serum 50μl |
| Sample Type | Serum, Plasma |
| Sensitivity | High |
| Applications | Medical diagnosis |
Benefits:
Storage and Stability:
Safety Information:
For the most accurate and detailed information, always refer to the specific product manual provided with the Calprotein Assay Kit you are using.

|
|
SARS-CoV-2 S1-RBD IgG Quant kit Quantitative Human Diagnostic Detect Reagent Kit Images |